Update on recently published USP standards for water used for nonsterile compounding, reconstitution, and cleaning of pharmacy equipment and utensils.
As expected, USP published their update to USP GC <795> Pharmaceutical Compounding – Nonsterile Preparations. You can download a copy of General Chapter here. While it remains to be seen how the State Boards of Pharmacy, the FDA, and the industry interpret and enforce the new standards, here are the relevant facts.
The Facts
- As stated in section USP GC <795>, Section 1.1 Scope, “Reconstitution of a conventionally manufactured nonsterile product in accordance with the directions contained in the manufacturer approved labeling is not required to meet the standards in this chapter.”
- The water quality standards for reconstitution of conventionally manufactured nonsterile products will be left to each drug manufacturer, or Board of Pharmacy to determine if otherwise not specified.
- Water used for compounded nonsterile preparations (CNSPs) must meet the water quality parameters spelled out in USP GC <1231>
- The rinsing of all equipment & utensils should comply with the water quality parameters spelled out in USP GC <1231>
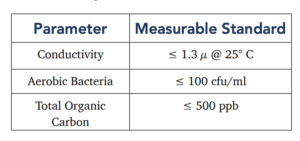
Water Quality Standards for Purified Water as Defined by USP
Our Actions
With USP leaving the topic of quality standards for reconstitution to the drug manufacturers themselves to decide, this appears to be a gray area. We continue to seek more guidance from regulatory bodies on what, if any, standards will apply should a manufacturer simply specify “water”, which most do. The consensus from the Boards of Pharmacy has been that pharmacists should use their professional judgement and if otherwise not specified, best practice would be to use purified or distilled water. Clearly, if your pharmacy is preparing CNSPs (Magic Mouthwash preparations, Tamiflu compounds, Prilosec or Prevacid preparations, come to mind) then adherence to USP GC <1231> Purified Water quality standards is now required and will most likely be scrutinized.
Moving Forward
Our recommendation from a quality, safety, and uniformity standpoint, is for pharmacies to meet the water quality standards laid out in USP GC <795> and USP GC <1231> whether they feel it is necessary or not. This leaves nothing to chance. The heightened bacterial and conductivity controls achieved with the additional filtration create a consistent, uniform product whether you’re reconstituting, preparing a nonsterile compound, or cleaning equipment. The enhanced filtration has the added benefit of providing greater peace-of-mind to pharmacy staff and customers should poor municipal water quality concerns continue to arise.
Be sure to mention you saw this article when you call.
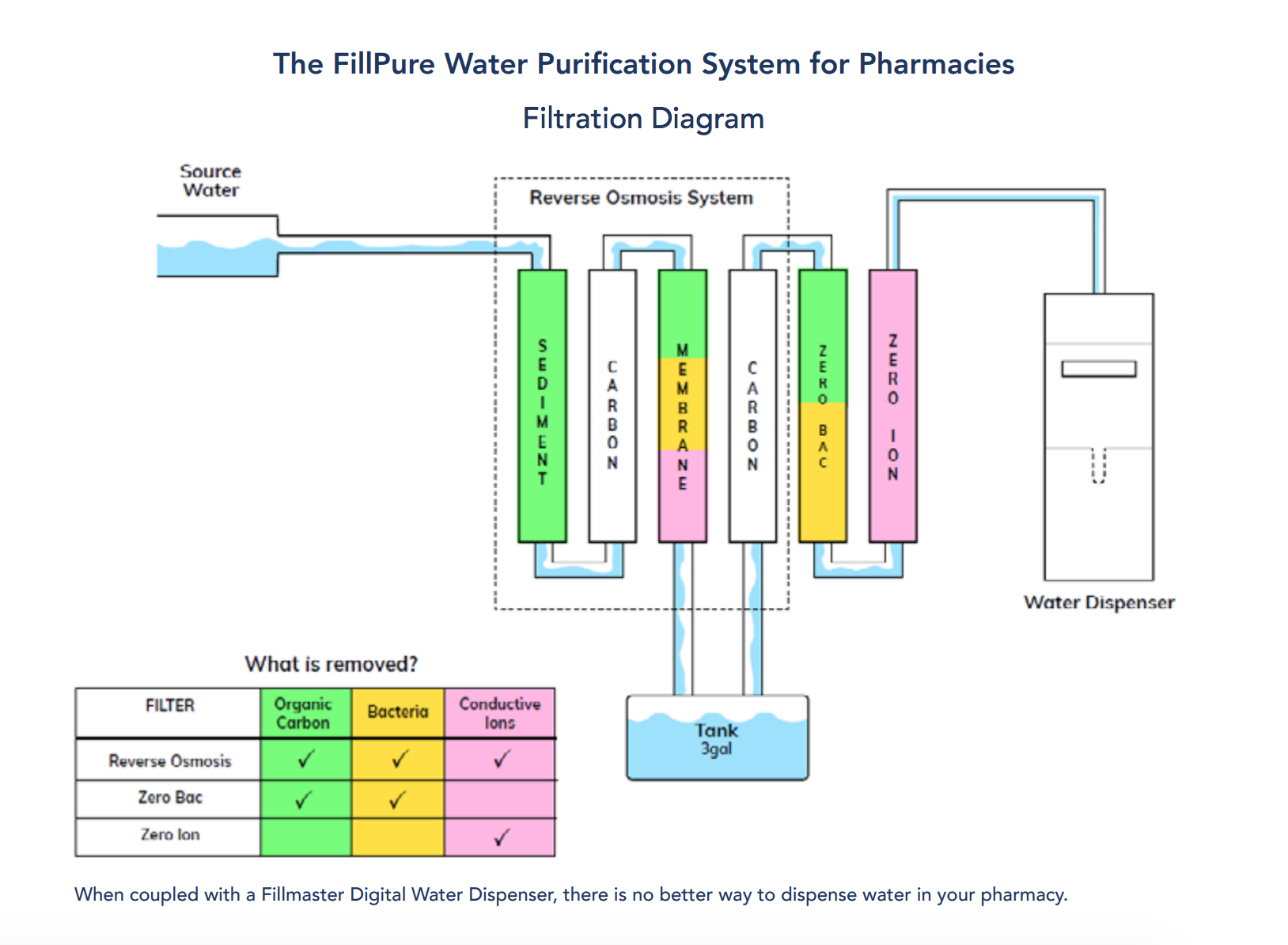
- Meet/exceed USP standards for Purified Water as defined by USP <795> and USP <1231>
Provide water of uniform quality and consistency for non-sterile compounding and reconstitution - To accomplish this, we built a multi-stage water filtration and purification system from the ground up, specifically with the needs of retail pharmacies in mind. Our extensive knowledge of source water quality across the U.S. led us to a solution that consistently produces uniform, high-quality water, provided your influent water meets or exceeds EPA guidelines for potable drinking water.
When coupled with a Fillmaster Digital Water Dispenser, there is no better way to dispense water in your pharmacy.